Microbial Pathogenesis
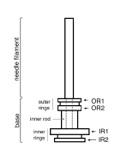
The topology of the needle complex
Recent crystallographic analyses of individually separate domains, which are predicted to be located within the plasma, revealed a common structural motif organized in repeating modules. Attempts have been made to “dock” these protein domains into the needle complex structure, which resulted in several mutually incompatible locations. We have used a combination of methods including bacterial genetics, biochemistry, mass spectrometry, site-specific nanogold labelling, and cryo electron microscopy/single particle analysis to experimentally determine the position of specific protein domains within the needle complex. In addition, we have identified specific sites of interaction among components of the needle complex, which are critical for stable assembly and the subsequent functional complex. Jointly, this analysis provides the first experimentally validated topographic map of different components of the needle complex of the S. typhimurium TTSS.
Schraidt, O., et al (2010).
Topology and organization of the Salmonella typhimurium type III secretion needle complex components.
PLoS Pathog. 6(4):e1000824 (abstract)
Technical highlights:
- selective disassembly of complexes
-
-nano-gold labelling at polyhistidine tags intorduced at N- or C-termini as well as internally
-
-visualization of nanogold labels by cryoEM
-
-X-linking of complexes and identification of cross-linked peptides by high-resolution mass spectrometry
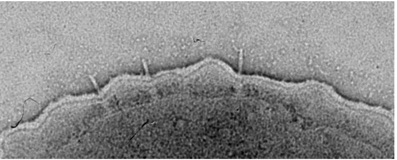
The needle filament
Efficient effector protein translocation is known to occur only after host cell contact. Therefore, it is conceivable that the extracellular filament is a key player in the transmission of this information, probably due to small conformational changes throughout the filament. This hypothesis is supported by mutations found in the homologous Shigella needle filament, which convert the system into a constitutively “on” state. If this is true, it would be justifiable to presume that the filament is provided with a certain degree of structural heterogeneity in order to accommodate the required conformational plasticity for signal transmission. Therefore, we analyzed the structure of the needle filament by cryo electron microscopy and discovered that the structure is, indeed, highly variable.
Galkin, VE. et al (2010).
The structure of the Salmonella typhimurium type III secretion system needle shows divergence from the flagellar system.
J Mol Biol. 396(5):1392-7 (abstract)
Technical highlights:
- isolation of long needle filaments
- cryo electron microsopy and helical reconstruction
Selected publications
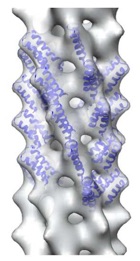
Conformational changes during assembly - the reprogramming phase
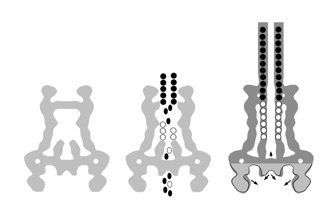
Assembly of the injectisome is a step-wise process during which stable intermediate substructures are being formed. While in the initial phase, the assembly is dependent on the cellular sec-machinery, it then proceeds in a sec-independent manner. During this later phase the base becomes already ‘secretion’ active and recognizes the inner rod protein PrgJ and the needle filament PrgI to be recruited into the complex. Once the needle filament reaches a certain lenght, the needle complex switches its substrate specificity (repgrogramming phase) and recognizes later substrates, such as the translocon or effector proteins. This change in substrate specificity highly correlates with large conformational changes indicating a structural basis for substrate recognition.
Marlovits, TC. et al (2004).
Structural insights into the assembly of the type III secretion needle complex.
Science. 306(5698):1040-2 (abstract)
Technical highlights:
- isolation of assembly intermediates
-
-cryo electron microsopy and single particle reconstruction
-
-selective disassembly of the needle filament
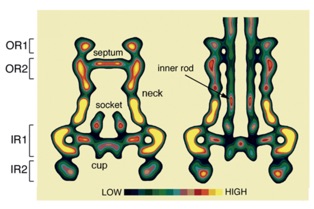
The needle length control
How to stop a polymerization event ? - or, When does the needle stop to grow ? The finding that the needle complex recruits the protein PrgJ as a new structural element located within the needle complex, prompted us to investigate its role during assembly. We hypothesize that the inner rod and the needle filament elongate as two separate substructures simultaneously. Completion of the inner rod induces a conformational change at the cup, resulting in a change in substrate specificity: as PrgI will no longer be recognized, the needle “stops” to grow.
Marlovits, TC. et al (2006).
Assembly of the inner rod determines needle length in the type III secretion injectisome.
Nature. 441(7093):637-40 (abstract)
Technical highlights:
-
-change of PrgI:PrgJ levels resulting in different lengths of the needle filaments
-
-cryo electron microsopy and single particle reconstruction
The export apparatus
The ‘export apparatus’ is a collection of highly conserved, polytopic inner membrane proteins. Using a biochemical and structural approach, we were able to demonstrate that the export apparatus proteins nucleate the coordinated assembly of the needle complex (‘inside-out’ assembly) and that the proteins are involved in the formation of the socket/cup structure, which is localized in the center of the needle complex.
Wagner, S.,et al (2010).
Organization and coordinated assembly of the type III secretion export apparatus.
Proc Natl Acad Sci U S A. 107(41):17745-50 (abstract)
Technical highlights:
- quantitative analysis of complexes by blue-native gel electrophoresis
- temporal expression of sub-components to determine the assembly pathway
-
-cryo electron microscopy and single particle analysis of mutant complexes
In particular, many Gram-negative pathogens such as Salmonella, Yersinia, Pseudomonas, enterohaemorrhagic E. coli (EHEC) or Shigella share common strategies to infect and colonize animal and plant hosts, resulting in a multitude of diseases ranging from diarrhea, typhoid fever, bubonic plaque to fire blight in fruit trees. They utilize the type III secretion system (TTSS) to initiate infection in eukaryotic cells.
In contrast Mycobacteria secrete bacterial proteins via the type VII (T7SS), which plays a key role in the infection of human host cells to cause tuberculosis. According to the World Health Organization, tuberculosis rates among the top three infectious diseases (including malaria and HIV) leading to fatal outcomes in humans.

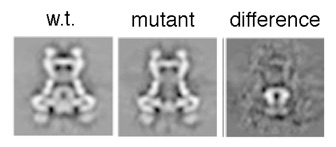
The blueprint of the needle complex at subnanometer resolution
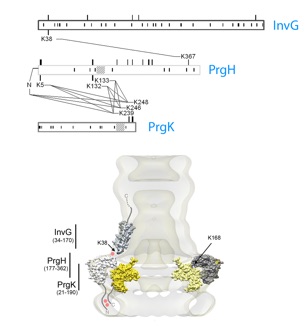
Assembly of the needle complex requires the precise arrangement of all the components involved. Using cryo electron microscopy and single particle reconstruction we determined the structure of the needle complex to sub-nanometer resolution. We found that the three major base proteins - PrgH, PrgK, and InvG - assemble into ring-like structures displaying an absolute stoichiometry of 24:24:15, resulting in an overall three-fold symmetry. Subsequently , at this resolution, we were able to unequivocally dock all atomic structures of the base proteins available into the electron microscopy map, which finally allowed us to determine the handedness of the complex and interpret the map at near atomic resolution. The map was experimentally validated by site-specific mutations at the predicted interaction sites.
Schraidt, O. and Marlovits, TC (2011).
Three-dimensional model of Salmonella’s Needle Complex at Subnanometer Resolution.
Science 331:1192-95 (abstract)
Technical highlights:
- selective disassembly of the complex into sub-structures
- change of preferred particle orientation by protonated poly-histidine tags
-
-cryo electron microscopy and single particle analysis of mutant complexes to subnanometer resolution
-
-functional validation of structural model by mutating predicted interaction sites
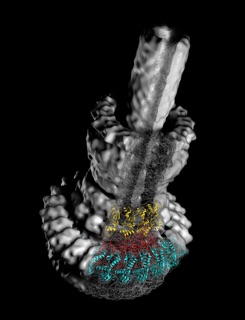
@ Kulcsar/Marlovits


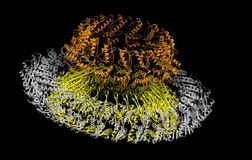
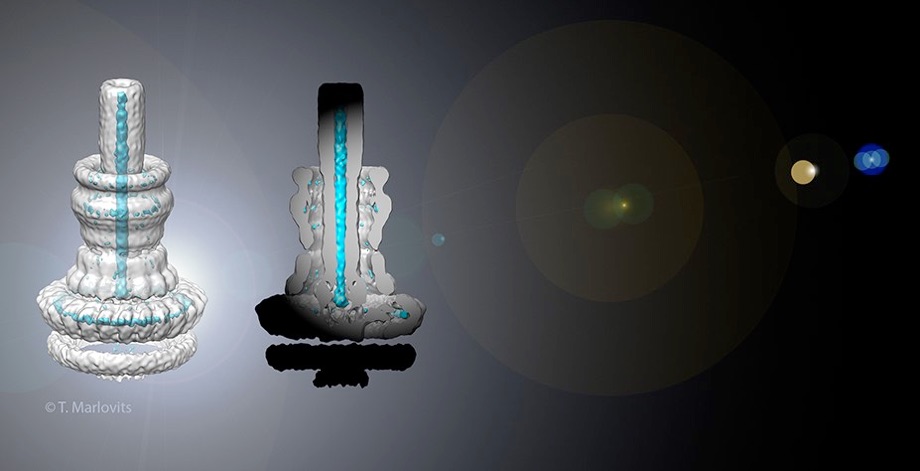
Injectisomes in action
The hallmark function of all type-3 secretion system is the transport of proteins across membranes. Here we have determined requirements for interaction with, transport through and exit from pathogenic injectisomes and designed novel substrates that become trapped during transport. Our structural analysis by cryo electron microscopy and single particle analysis revealed for the first time the secretion path spanning ~800 Angstroem across two membranes and the periplasmic space. We showed that specifically designed substrates are transported in a polarized fashion, starting with the insertion of the N-terminal domain into the membrane embedded needle complex. By using cryo electron tomography, we elucidate unperturbed type-3 secretion systems in action in situ. This study represents the first structural analysis of unfolded protein transport across several membranes in situ.
Radics, J., Königsmaier, L., and Marlovits, TC
Structure of a pathogenic type-3 secretion system in action.
Nature Structural & Molecular Biology (DOI 10.1038/nsmb.2722)
Technical highlights:
- design of novel type-3 substrates, which become trapped during transport
- cryo electron tomography of type-3 secretion systems
-
-cryo electron microscopy and single particle analysis of substrate-trapped injectisomes
-
-visualization of unfolded protein transport across membranes
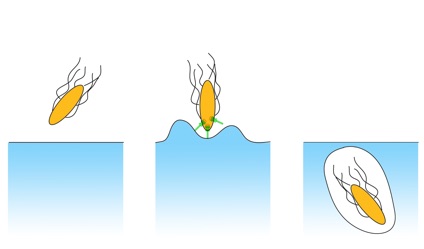
host cell
approaching
toxin injection
engulfment
One of the most exciting discoveries in the last few years is that supramolecular assemblies are one of the key elements involved in the infection of eukaryotic cells by bacterial pathogens. These systems give rise to intimate contact between cells, deliver specific toxins - which are collectively known as effectors proteins - into host cells, and possess the remarkable ability to modulate diverse regulatory networks.
Mycobacterial Type-7 Secretion System visualized for the first time
Mycobacteria are characterized by their impermeable outer membrane that is rich in mycolic acids. To transport substrates across this complex cell envelope, mycobacteria rely on type VII (also known as ESX) secretion systems. In Mycobacterium tuberculosis, these ESX systems are essential for growth and full virulence and therefore represent an attractive target for anti-tuberculosis drugs. However, the molecular details underlying type VII secretion are largely unknown, due to a lack of structural information. Here, we report the molecular architecture of the ESX-5 membrane complex from Mycobacterium xenopi determined at 13 Å resolution by electron microscopy. The four core proteins of the ESX-5 complex (EccB5, EccC5, EccD5 and EccE5) assemble with equimolar stoichiometry into an oligomeric assembly that displays six-fold symmetry. This membrane-associated complex seems to be embedded exclusively in the inner membrane, which indicates that additional components are required to translocate substrates across the mycobacterial outer membrane. Furthermore, the extended cytosolic domains of the EccC ATPase, which interact with secretion effectors, are highly flexible, suggesting an as yet unseen mode of substrate interaction. Comparison of our results with known structures of other bacterial secretion systems demonstrates that the architecture of type VII secretion system is fundamentally different, suggesting a novel secretion mechanism.
Beckham K.S.H et al. Structure of a mycobacterial type VII secretion system membrane complex.
Nature Microbiology, 2017. DOI: 10.1038/nmicrobiol.2017.47.
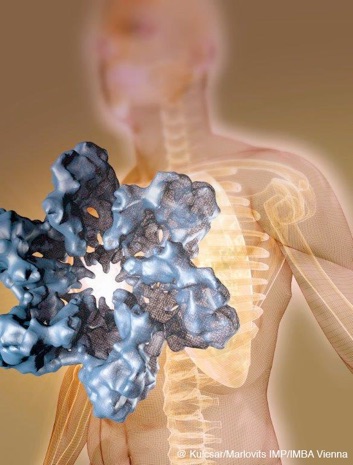
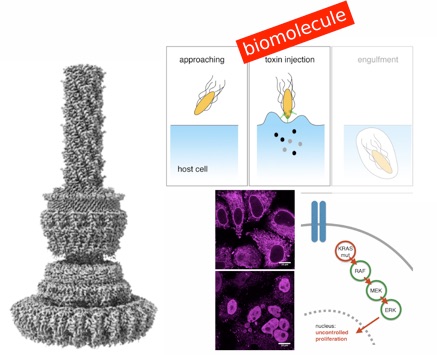
Type-3 Secretion System exploited for novel therapeutic strategies
Protein-based affinity reagents (like antibodies or alternative binding scaffolds) offer wide-ranging applications for basic research and therapeutic approaches. However, whereas small chemical molecules efficiently reach intracellular targets, the delivery of macromolecules into the cytosol of cells remains a major challenge; thus cytosolic applications of protein-based reagents are rather limited. Some pathogenic bacteria have evolved a conserved type III secretion system (T3SS) which allows the delivery of effector proteins into eukaryotic cells. Here, we enhance the T3SS of an avirulent strain of Salmonella typhimurium to reproducibly deliver multiple classes of recombinant proteins into eukaryotic cells. The efficacy of the system is probed with both DARPins and monobodies to functionally inhibit the paradigmatic and largely undruggable RAS signaling pathway. Thus, we develop a bacterial secretion system for potent cytosolic delivery of therapeutic macromolecules.
Chabloz et al. Salmonella-based platform for efficient delivery of functional binding proteins to the cytosol
Communications Biology, 2020. DOI: 10.1038/s42003-020-1072-4
Type-3 Secretion System in Action
Many bacterial pathogens rely on virulent type III secretion systems (T3SSs) or injectisomes to translocate effector proteins in order to establish infection. The central component of the injectisome is the needle complex which assembles a continuous conduit crossing the bacterial envelope and the host cell membrane to mediate effector protein translocation. However, the molecular principles underlying type III secretion remain elusive. Here, we report a structure of an active Salmonella enterica serovar Typhimurium needle complex engaged with the effector protein SptP in two functional states, revealing the complete 800Å-long secretion conduit and unraveling the critical role of the export apparatus (EA) subcomplex in type III secretion. Unfolded substrates enter the EA through a hydrophilic constriction formed by SpaQ proteins, which enables side chain-independent substrate transport. Above, a methionine gasket formed by SpaP proteins functions as a gate that dilates to accommodate substrates while preventing leaky pore formation. Following gate penetration, a moveable SpaR loop first folds up to then support substrate transport. Together, these findings establish the molecular basis for substrate translocation through T3SSs and improve our understanding of bacterial pathogenicity and motility.
Miletic et al. Substrate-engaged type III secretion system structures reveal gating mechanism for unfolded protein translocation
Nature Communications, 2021. DOI: 10.1038/s41467-021-21143-1


